9.1 Oxidation and Reduction
Redox reactions
There are three main types of reactions that occur in chemistry:
-Acid-Base reactions
-Precipitation reactions
-Redox reactions
A redox reaction involves two processes, reductions and oxidation.
Reduction and Oxidation reactions
Both reduction and oxidation can be considered in a number of ways, and all three descriptions have merit in their own right. The different ways of describing these processes are:
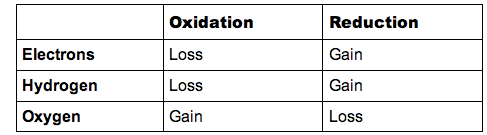
-In regards to specific elements - oxidation and hydrogen
-In terms of electron transfer
-In terms of oxidation number
Oxidation: Combining with oxygen


-The Statue of Liberty in New York, USA was restored in 1986 as it was found that corrosion had occurred between the wrought iron structural support and the outer copper skin.
-Shellac (a resin secreted by the female lac bug found on trees in Thailand and India) was originally inserted as an insulator between the copper and the iron but over time the insulation had failed and the iron supports rusted.
-The copper in the Statue of Liberty oxidized to form an outer green coating called the patina. When the restoration work on the Statue of Liberty was completed the statue was brown in colour. It has taken many years for it to oxidize fully and reform the patina (figure 1).
Reduction: Removal of oxygen or addition of hydrogen
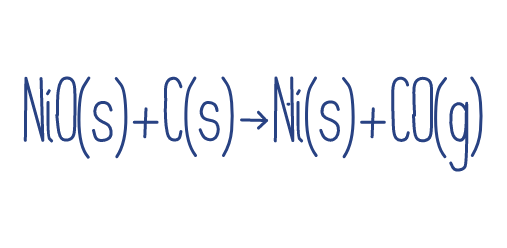
-In this reaction nickel(II) oxide is reduced by carbon to give metallic nickel
-Reduction may also be considered as the addition of hydrogen. For instance:
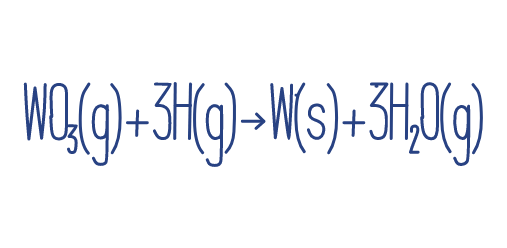
-This mirrors the previous interpretation of reduction, as oxygen is removed from Tungsten(VI) oxide in the process
Oxidation and Reduction in terms of electron transfer
Example 1: 2Mg(s) + O2
(g) 2MgO(s)
Example 2: 2Br- (aq) + Cl2 (aq) Br2 (aq) + 2Cl- (aq)
If a substance loses electrons then something else must be gaining electrons. The gain of one or more electrons is called reduction. In the first example oxygen is reduced as it is gaining two electrons from magnesium to form the oxide ion O2- . Similarly, in the second example chlorine is reduced as each chlorine atom gains one electron from a bromide ion to form a chloride ion. Since the processes involve the transfer of electrons oxidation and reduction must occur simultaneously. Such reactions are known as redox reactions. In order to distinguish between the two processes half-equations are often used:

Understanding that magnesium must lose electrons and oxygen must gain electrons when magnesium oxide MgO is formed from
its elements is a good way to remember the definitions of oxidation and reduction. However, some students prefer to use the mnemonic
OILRIG: Oxidation Is the loss of electrons and r eduction Is the gain of electrons.
Big Picture
Oxidation number rules
1. In an ionic compound between two elements the oxidation
state of each element is equal to the charge carried by the
ion
Na+Cl- (Na = +1 ; Cl = -1 )
2. For covalent compounds assume the compound is ionic with the more electronegative element Forming the negative ion
CCl4 (C = +4; Cl = -1 )
3. The algebraic sum of all the oxidation states in a compound = zero
CCl4
[(+4) + 4 x (-1 ) = 0]
4. The algebraic sum of all the oxidation states in an ion = the charge on the ion
SO4
2-
[(+6) + 4 x (-2) = -2]
6. Elements not combined with other elements have an oxidation state of zero
e.g. O2
; P4
; S8
.
7. Oxygen when combined always has an oxidation state o -2 except in peroxides (e.g. H2O2 ) when it is -1 .
8. Hydrogen when combined always has an oxidation state o +1 except in certain metal hydrides (e.g. NaH) when it is -1.
9..Many elements can show different oxidation states in different compounds. When elements show more than one oxidation state the oxidation number is represented by using Roman numerals when naming the compound, e.g. FeCl2 iron(II) chloride.
Oxidation States
When an element is oxidized its oxidation state increases,
e.g. Mg(s) --> Mg2+ (aq) + 2e-
Oxidation numbers before reaction
Mg: 0
Oxidation numbers after reaction
Mg2+: 2+
When an element is reduced its oxidation state decreases,
e.g. SO42- (aq) + 2H+ (aq) + 2e- --> SO32- (aq) + H2O(l)
Oxidation numbers before reaction
SO42-: +6
Oxidation numbers after reaction
SO32-: +4
The change in the oxidation state will be equal to the number of electrons involved in the half-equation. Using oxidation states makes it easy to identify whether or not a reaction is a redox reaction.
Not redox reactions (no change in oxidation states)
precipitation: Ag+ (aq) + Cl- (aq) --> AgCl(s)
Oxidation numbers before reaction
Ag+: +1
Cl-: -1
Oxidation numbers after reaction
AgCl(s): (+1)(-1)
Variable Oxidation States
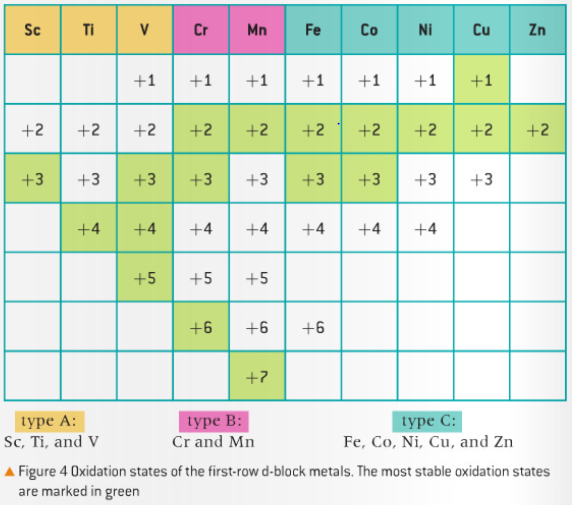
-As mentioned in the rules assigning oxidation states above, although many elements have fixed oxidation states, in their ions and compounds, such as the group 1 alkali metals and the group 2 alkaline earth metals, variable oxidation states exist for many main-group non-metals and in particular for most of the transition elements. What is special is that variable oxidation states are a characteristic property of the transition metals. The range of oxidation states for the figure below is demonstrated in the d-block elements below, which is in section 14 of your IB data booklet! Make sure you remember this for your exam. Not to mention, IUPAC describes transition elements whose atoms have an incomplete d-subshell. As you can see in the first row, d-block elements, the transition elements are Sc to Cu inclusive.
Oxidizing and Reducing agents:
An Oxidizing agent causes another species to be oxidized, and is itself reduced in the process. A reducing agent causes another species to be reduced, and is itself oxidized in the process.

Here is a video to help you prepare for your IB exam by gaining an even stronger understanding of oxidizing and reducing agents.
Balancing Redox Equations
Generally balancing chemical equations is pretty straightforward as you would only need to consider the conservation of atoms. However, because electrons are involved with redox reactions, what now must be considered is the conservation of change along with the conservation of atoms when balancing redox reactions.
Half reaction method
Split the redox reaction into two half reactions
1. Split the redox reaction into two half reactions
2. Balance all atoms except for hydrogen and oxygen
3. Balance oxygen by adding water
4. Balance hydrogen by adding H+
5. Balance the charge using e-
6. Add OH to use up H+ if in a basic medium
Balance this redox reaction by using the half reaction method.
Fe2+ + Cr --> Fe + Cr3+
Solution using the half reaction method
We start by writing the two half reactions. Chromium is being oxidized, and iron is being reduced:
Cr --> Cr3+ oxidation
Fe2+ --> Fe reduction
Then we include the appropriate number of electrons on the proper side to balance the charges for each reaction:
Cr --> Cr3+ + 3e−
Fe2+ + 2e− --> Fe
The first reaction involves three electrons, while the second reaction involves two electrons. The least common multiple of these two numbers is six, so to get six electrons in each reaction we need to double the first reaction and triple the second one:
2 × [Cr --> Cr3+ + 3e−] = 2 Cr --> 2 Cr3+ + 6e−
3 × [Fe2+ + 2e− --> Fe] = 3 Fe2+ + 6e− --> 3 Fe
We can combine the two final reactions, noting that the electrons cancel:
2 Cr + 3 Fe2+ + 6e− --> 2 Cr3+ + 3 Fe + 6e−
The overall, balanced redox reaction is
2 Cr + 3 Fe2+ --> 2 Cr3+ + 3 Fe
Once you have mastered the half-reaction method, try the oxidation reaction and it may be an easier method for you!
Oxidation number method
Assign oxidation numbers to identify redox half reactions
1. Assign oxidation numbers to identify redox half reactions
2. Determine the total number of electrons transferred for the oxidation half reaction and
reduction half reaction
3. Find the lowest common multiple between the number of electrons gained and lost from the
half reactions and multiply the reactants accordingly
4. Balance atoms of the oxidized and reduced elements in products
5. Balance the other atoms
6. Balance oxygen by adding water
7. Balance hydrogen by adding H+
8. Add OH to use up H+ if it is basic condition
Try a practise problem and see if you get the right answer!
Balance CS2(g) + O2(g) --> CO2(g) + SO2(g)
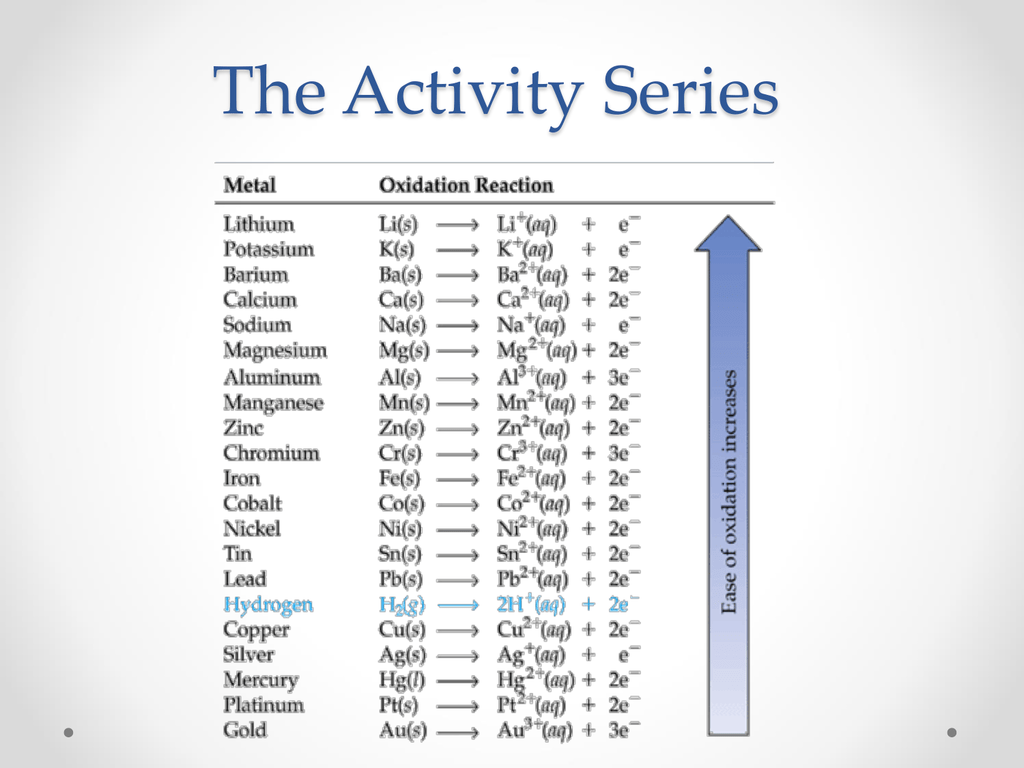
The Activity Series
The series ranks metals according to the ease with which they undergo oxidation
Reactive metals towards the top
Hydrogen(non - metal) is included due unique properties to act as one(highlighted in blue)
Examples:
As shown in the example above, since zinc is higher up on the activity series than hydrogen, it is able to displace the hydrogen ions and react with the chloride forming zinc(ii) chloride
Likewise, since aluminum is higher on the activity series than iron, it is able to displace it to react with oxygen forming aluminum oxide
Reactive Series
Opposite of the activity series, the reactivity series deals with reactions regarding the group 17 elements. In their case, smaller atomic radius and higher electronegativity means an increase in reactivity as evident from the graph.
Example:
In this case, since chloride has a smaller radius and is more electronegative than bromine, it thus is reduced and gains electrons(from potassium) to form its ions. Bromine and the other hand hence oxidizes and loses electrons(from potassium). Therefore, on the surface of all the redox theory, that's how chloride is able to displace bromine to form potassium chloride
Use of chloride and ozone as disinfectants in drinking water
Water supplies are usually cleansed through strong oxidizing agents such as chlorine (Cl2) or ozone (O3) to kill microbial pathogens
It can be added in three forms: chloride gas(Cl2), sodium hypochlorite (NaOCl), and calcium hypochlorite (Ca(OCl)2). All three solutions produce hypochlorous acid (HOCl) which is the antibacterial agent
However, chloride may cause problems for individuals. Some people dislikes the taste of its odor, and Chlorine can also react with other chemical to form toxins such as chloroform (CHCl3)
Redox Titration Reactions
Formula recalls:
Example:
Consider the following balanced equation for the reaction of potassium manganate(VII) with ammonium iron(II) sulfate.
5Fe2+(aq) + MnO4(aq) + 8H+(aq) → 5Fe3+(aq) + Mn2+(aq) + 4H2O(l)
In a titration, 0.0280 dm3 of the potassium manganate(VII) solution reacted completely with 0.0250dm3 of a 0.0100 mol dm-3 solution of ammonium iron(II) sulfate. Determine the concentration, in g dm-3, of the potassium manganate(VII) solution
Solution:
Step 1: Balanced the redox equation with oxidation states(has been done)
Step 2: Organize the given data
Va = volume of Fe2+ = 0.0250 dm3
Ca = concentration of Fe2+ = 0.0100 mol dm-3
Vb = volume of MnO4- = 0.0280 dm3
Cb = concentration of MnO4- = the unknown
Va = stoichiometry coefficient = 5
Vb = stoichiometry coefficient = 1
Step 3: Substitute into the equation
Step 4: Solve
Step 5: Cb = 0.00179 mol dm-3
Step 6: To convert dm-3 to gdm-3 we multiply the concentration by the substance's molar mass
Environmental Application Of Redox Chemistry: The Winkler Method
The method is used to measure the amount of oxygen dissolved in water to support the living of aquatic organisms. Since O2 is non-polar but water is polar, we can expect the amount of oxygen dissolved to be very low
Note the solubility is inversely proportional to temperature(one increases as the other decreases and vice-versa)
Quantitating the amount of oxygen dissolved in water also says a lot about the pollution level(more dissolved = less pollution)
The degree of organic pollution in a sample of water can be quantitated by the biochemical oxygen demand(BOD) measured in ppm(parts per million)
Formula Of ppm:

Typical biological oxygen demands for water samples:
How BOD Is Affected
Organic matter is discharged into water and becoming food for bacterias
Bacteria breaks it down into through series of oxidation reactions into CO2 and H2O
As the bacteria multiplies, more oxygen is dissolved for the oxidation reactions
Hence if rate of oxidation is > rate of oxygen dissolved
If BOD > dissolved oxygen aquatic life dies
Substances Produced By Bacteria Under Aerobic And Anaerobic Conditions
Measuring BOD using the Winkler Method
With this method, an iodine/thiosulfate redox titration is performed to measure the dissolved oxygen in a water sample
The series of reactions:
In a 0.0500 dm3 sample of water, if 0.00525 dm3 of a 0.00500 mol dm-3 solution of sodium thiosulfate causes it to react with the iodine produced,
Find the concentration of dissolved oxygen, in ppm, in the water sample
Find the BOM assuming the max solubility of oxygen is 9.00 ppm and the temperature constant
Define the significance of the BOM value
Solution:
Step 1: Derive the Balanced redox ratio from the given 3
Step 2: Organize the given date
Va = volume of sodium thiosulfate = 0.00525 dm3
Ca = concentration of sodium thiosulfate = 0.00500 mol dm-3
Vb = volume of Oxygen = 0.0500 dm3
Cb = concentration of Oxygen = the unknown
Va = stoichiometry coefficient = 4
Vb = stoichiometry coefficient = 1
Step 3: Substitute into equation
Cb = 1.31 x 10^-4 mol dm-3
Part 2: Find BOM in ppm
Step 5: To convert dm-3 to gdm-3 we multiply the concentration by the substance's molar mass
Note that:
- 10^-3 g dm-3 = mg dm-3
- mg dm-3 = ppm
Step 6: Find the BOM
With the Concentration of oxygen determined, all we need to do is subtract it from the maximum solubility which 9 ppm is the given
BOM = 9.00 - 4.19 = 4.81ppm
Part 3:
The BOM value of the sample of water suggests it's organic pollution level is around the level of untreated domestic sewage(refer back to the typical biological oxygen demands charts), thus it is recommended a sewage system is installed
This video really reinforces our understanding of redox titrations
